IHC Protocol
IHC identifies the expression pattern of a protein in tissue through specific antibody binding. So the tissue used for IHC is often preserved and fixed to retain localization of the protein. The protocols included here are mainly for these formalin-fixed, paraffin-embedded tissues (IHC-P), which offer superior tissue/cell morphology, but require antigen retrieval before staining. In addition to the general protocol, we also include here the step-by-step mouse-on-mouse and human-on-human IHC protocols.
» Find IHC Antibodies (browse all)
IHC for paraffin-embedded tissue
Solutions and reagents
Protocol
Solutions and reagents
1. Xylene
2. Ethanol, anhydrous denatured, histological grade (100%, 95%, 85%, 75%)
3.Washing buffer:
| 1XPBST | 1 L |
| 10X PBS | 100 mL |
| dH2O | 900 mL |
| Tween-20 | 1 mL |
| 10X PBS | 1 L |
| NaH2PO4•2H2O | 2.84 g |
| Na2HPO4•12H2O | 27.2 g |
| NaCl | 90 g |
| dH2O | 1000 mL |
The pH should be about 7.2. Adjust if necessary with 1 M NaOH or 1 M HCl
4. Antigen Retrieval Solution:
A. 10mM Sodium Citrate Buffer, pH 6.0
| C6H8O7•H2O | 0.21g |
| C6H8Na3O7•2H2O | 0.21g |
| dH2O | 1000 mL |
Adjust pH to 6.0
B. 10mM Tris Buffer with 1mM EDTA,pH 8.0 or 8.5 or 9.0
| Tris | 1.21 g |
| EDTA | 0.37 g |
| dH2O | 1000 mL |
Adjust pH* to 8.0 or 8.5 or 9.0
*Note: Please refer to the antibody for individual antigen retrieval buffer and working conditions.
5. 3% Hydrogen Peroxide
6. Hematoxylin QS
7. Permanent Mounting Medium
Protocol
A. Deparaffinization and Rehydration
- Heat slides in an oven at 60 °C for 5 min.
- Wash slides 3 times for 10 min each in xylene.
- Wash slides in 95% ethanol, 1 min.
- Wash slides in 85% ethanol, 1 min.
- Wash slides in 75% ethanol, 1 min.


- Rinse slides for 5 min in distilled water.
B. Antigen Retrieval*
- Put the slides in Antigen Retrieval Solution and keep 120℃ for 2.5 min in pressure cooker.
- Then cool down at room temperature.
- Wash slides three times with distilled water (2 min each).
*Note: Please refer to the antibody for individual antigen retrieval buffer and working conditions.
C. Staining

- Inactivate endogenous peroxidase by covering tissue with 3% hydrogen peroxide for 15 min.

Wash slides twice with 1X PBST (2 min each).
- Dilute primary antibody in the IHC Antibody Diluent per recommendation on the data sheet.
- Apply primary antibody to each section and incubate 90 min at room temperature*. Make sure the primary antibody solution covers the tissue evenly.
*Note: Please refer to the antibody for individual buffer and working conditions.

Wash slides three times with 1X PBST (2 min each).

- Apply to each section secondary antibody and incubate for 15 min at room temperature*.
*Note: Please refer to the antibody for individual buffer and working conditions.

Wash slides three times with 1X PBST (2 min each).

- Add freshly prepared DAB substrate to the sections.
- Incubate tissue sections with the substrate at room temperature until suitable staining develops (generally about 5 min).

Rinse sections with water.

- Counterstain with Hematoxylin QS for 3 min

Rinse sections with water.
- Wash slides in 75% ethanol, 1 min
- Wash slides in 85% ethanol, 1 min
- Wash slides in 95% ethanol, 1 min
- Wash slides in 2 changes of 100% ethanol rinses, 1 min each
- Wash slides in 3 changes of xylene, 1 min each


- Mount coverslips on slides using Permanent Mounting Medium

- Allow slides to dry overnight at room temperature and then analyze the results with microscope
Example
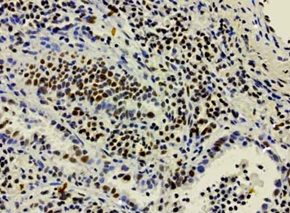
IHC staining of OriGene’s MKI67 UltraMAB (clone UMAB107) of normal human tonsil tissue. (UM500008).
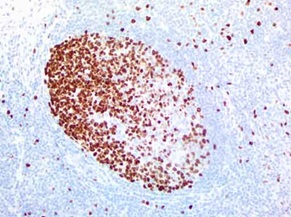
IHC staining of OriGene’s MKI67 UltraMAB (clone UMAB107) of normal human tonsil tissue. (UM800033).
Useful links:
View our list of over 140,000 human tissues
View our list of the Specific Antibodies for Anatomic Pathology
View our list of detection kits developed by GBI Labs, Inc


 United States
United States
 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China
