Human MAGEA3 ELISA KIT (1 x 96 wells)
CNY 4,700.00
货期*
3周
规格
Specifications
| Product Data | |
| Description | Human MAGEA3 ELISA KIT (1 x 96 wells) |
| Size | 1 x 96 wells |
| Format | 8x12 divisible strips |
| Assay Type | Sandwich |
| Assay Length | 4 hours incubations; 1 hour washing and analyzing samples |
| Signal | Colorimetric |
| Curve Range | 62.5pg/ml-5000pg/ml |
| Sample Type | Human serum, plasma and other biological fluids. |
| Sample Volume | 100µl |
| Specificity | This kit is used for quantitative detection of Human MAGEA3 |
| Sensitivity | 12.9pg/ml |
| Reactivity | Human |
| Cross Reactivity | Recombinant human MAGEA6 cross-reacts approximately 10.8 % in this assay. There is no significant cross-reactivity with other relevant proteins. |
| Interference | No significant interference observed with available related molecules. |
| Components |
|
| Background | Melanoma-associated antigen 3 (MAGE-A3) is broadly expressed in a variety of malignancies such as breast cancer, melanoma, head and neck cancer, lung cancer, gastric cancer, colorectal cancer, and prostate cancer. It has been shown that MAGE-A3 expression is associated with cancer progression and metastasis. In addition, increased expression of MAGE-A3 in cancer cells is associated with poor prognosis of cancer patients. |
| Gene Symbol | MAGEA3 |
| Standard Curve |
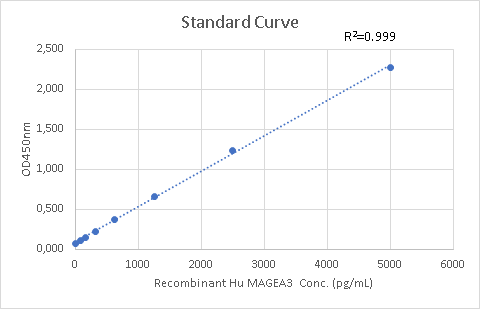
Data image for Human MAGEA3 ELISA KIT.
|
Documents
| Product Manuals |
Customer
Reviews
Loading...


 United States
United States
 Germany
Germany
 Japan
Japan
 United Kingdom
United Kingdom
 China
China

